Journal statistics 2022
Overall acceptance rate: 41%
Mean time to decision on initial submissions: 61 days Mean time to decision on resubmissions: 16 days Instructions for Authors Thank you for choosing to submit your manuscript for publication through the Rwanda Medical Journal (RMJ). These instructions will ensure that you understand which types of manuscripts the journal accepts and that your submission is correct and ready to move through peer-review, production, and publication process smoothly. Please take the time to read and follow the instructions as closely as possible. Doing so will ensure your paper matches the RMJ requirements for publication. |
AIMS AND SCOPE OF JOURNAL
The Rwanda Medical Journal (RMJ), is a Not-For-Profit scientific, medical, journal that is published entirely online in open-access electronic format (click here). The RMJ was created in 1967 and intends to be published indefinitely.
MISSION STATEMENT
The RMJ is an interdisciplinary research journal for publication of original work in all the major disciplines. Through a rigorous process of evaluation and peer review, The RMJ strives to publish original works of high quality for a diverse audience of healthcare professionals. The Journal seeks to deepen knowledge and advance scientific discovery to improve the quality of care of patients in Rwanda and internationally
The Rwanda Medical Journal (RMJ), is a Not-For-Profit scientific, medical, journal that is published entirely online in open-access electronic format (click here). The RMJ was created in 1967 and intends to be published indefinitely.
MISSION STATEMENT
The RMJ is an interdisciplinary research journal for publication of original work in all the major disciplines. Through a rigorous process of evaluation and peer review, The RMJ strives to publish original works of high quality for a diverse audience of healthcare professionals. The Journal seeks to deepen knowledge and advance scientific discovery to improve the quality of care of patients in Rwanda and internationally
Submit an article on our website
www.rwandamedicaljournal.org
www.rwandamedicaljournal.org
Originality
By submitting your manuscript to the RMJ it is understood that it is an original manuscript and is unpublished work and is not under consideration elsewhere. Plagiarism, including duplicate publication of the author's own work, in whole or in part without proper citation is not accepted by the journal. Manuscripts submitted to the journal may be checked for originality using plagiarism software.
The RMJ is a fully open-access journal which does not charge a submission fee and does not charge a publication fee. After publication, articles are free to access at bioline (here). We accept the following types of manuscripts:
1. Original research
2. Review articles
3. Methodology articles (e.g., articles describing a new surgical or practical technique)
4. Case reports (including images) and case-series
5. Editorials/opinion articles
6. Quality Improvement projects
7. Letters to the editor
Authors do not incur charges for submission or publication.
The RMJ is a peer-reviewed journal following a double “blind” peer-review model. This means that reviewers will not know your identity and likewise we will not provide you with details of our peer-reviewers. We will provide reviewers with your designation and country of employment to provide relevance to the manuscript. After initial screening by the chief-editor and the editorial team, which takes only a few days, manuscripts are reviewed by at least two independent peer-reviewers. The journal aims to give decisions on manuscripts within 6-8 weeks.
Submission language:
Only articles in English are accepted. Please note that the RMJ will only publish manuscripts in English. Poor English should not prevent acceptance provided the paper's content is of high scientific quality
Plagiarism
The editorial team uses plagiarism software to screen all submitted articles. Any related or discovered plagiarism disrupts the credibility of the article and leads to its immediate and full rejection. In cases where significant plagiarism is identified, the editorial team may, at their discretion, contact the line-manager (supervising clinician) of the offense.
Duplicate publication: Duplicate publication is publication of a paper that overlaps substantially with one already published, without clear, visible reference to the previous publication (ICMJE). When authors submit a manuscript reporting work that has already been reported in large part in a published article or is contained in or closely related to another paper that has been submitted or accepted for publication elsewhere, the letter of submission should clearly say so and the authors should provide copies of the related material to help the editor decide how to handle the submission.
Checklists
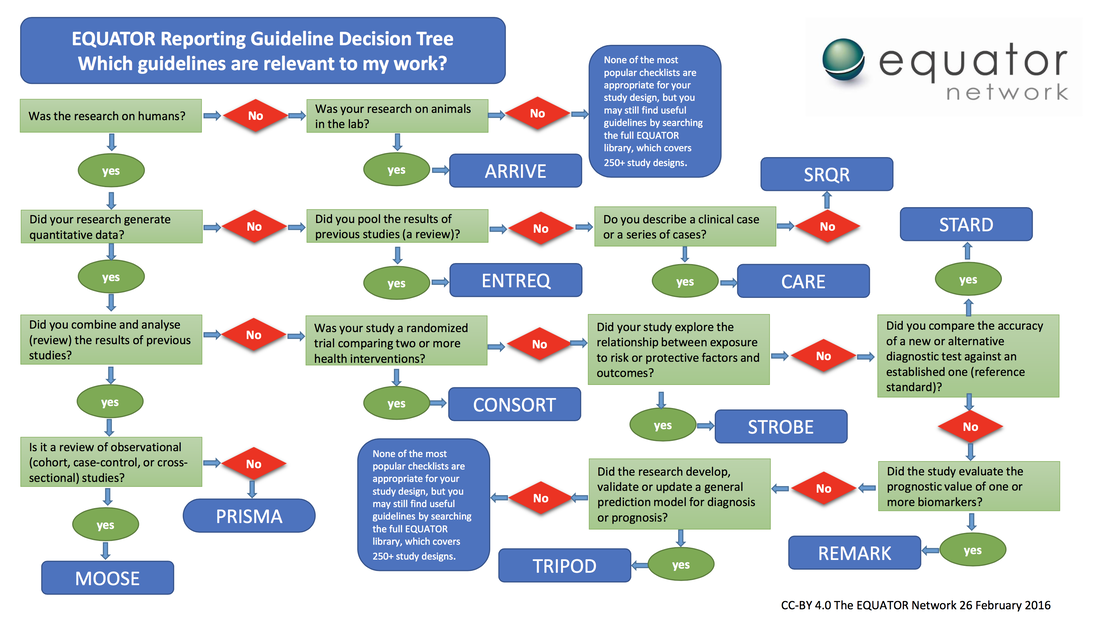
We strongly recommend that authors use a Checklist (reporting guideline) to review their manuscript, prior to submitting. Where possible state in your manuscript which checklist you have employed (more information is available from the Equator Network (http://www.equator-network.org/).
If you are unsure which reporting guildline to use then follow this decision tree below
0Manuscript types
The journal considers the following types of articles:
1. Original research:
These should be pieces of original research that have not been published in other peer-reviewed journals
Structure: The manuscript should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods, Results, Discussion, Conclusion (see writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 3500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum four produced to high quality.
Tables and figures should be presented in the text in Results section of the manuscript and should be single-spaced.
Trial registration and Institutional Review Board (IRB): The IRB reference code should be reported in all manuscripts as the last line of the manuscript abstract. The trial registration number should be included where relevant.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. STROBE
2. Reviews:
We accept the following types of review articles:
i. Systematic reviews and realist reviews
ii. In-depth critical reviews in areas where these are more appropriate; e.g., coverage of issues recently updated. For non-commissioned reviews, the editorial board recommend contacting us to discuss the concept before investing time into the manuscript
iii. Best-evidence topics (BETs). These are not systematic reviews but critical review articles of evidence related to a specific clinical question. Contact Dr. Peter Cartledge ([email protected]) for more details and a Word template.
Structure: the manuscript should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods (including search strategy where relevant), Results, Discussion, Conclusion (see writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 4500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum four produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
3. Case report or case studies:
These should be a well-described case for educational purposes and/or to extend scientific knowledge regarding unique clinical cases. These can contain unusual features of disease, treatment, transmission and/or control.
Structure: the manuscript should be divided into the following sections: Keywords, Short running Title, Abstract. Introduction, Case(s), Conclusions (see writing rules & styles below for more information).
Abstract: A short abstract (maximum 100 words) can be provided using the following two subheadings: Case, Discussion.
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 1500-2000 words (not including, title, tables, abstract and/or references)
Tables: Maximum 2
Figures: Maximum three produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
Consent: Informed consent must be documented. A signed written consent from the patient should be provided for any potentially identifiable images.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. CARE checklist
Clinical images:
Images are accepted and encouraged with the same instructions as case-reports and therefore they should include a description of the case (as above). Written consent should be obtained from patients and a copy of the consent form included with the submission.
4. Methodology articles:
New experimental methods, tests, techniques, or procedures. These should be completely new (novel) or improved, and/or compared with different methods
Structure: should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methodology, Conclusions (see Writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on number of references
Word limit: 3500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum 4 produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single spaced, each table and figure on a separate page, starting with a “page break”.
5. Editorial/Opinion articles:Editorials are short opinion papers.
These articles might mostly be object to critical and opened discussion categorized as ‘’commentaries‘’. These are normally commissioned and should always be discussed with the editorial board prior to starting work on the manuscript.
Structure: Short, narrow and focused
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on number of references
Word limit: 2500 words (including, references)
Tables and figures: Maximum 2 (e.g. 1 Table & 1 figure) produced to high quality. Tables and figures should be inserted in the text where they would appear in the manuscript. Do not attach as appendices.
6. Quality improvement project
Quality Improvement projects that have not been published in other peer-reviewed journals:
These should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods, Results, Discussion, Conclusion (see writing rules & styles below for more information). If a traditional IMRaD structure is not used then authors may choose to present in a PDSA (Plan, Do, Study, Act) format
Methodology: How was the QI project undertaken
Results: What was found and how did care improve
Discussion: What are the implications and potential for wider application.
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 3000 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum 4 - produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
Trial registration and Institutional Review Board (IRB): Quality Improvement projects do not require IRB review for publication. However authors should state that the project has been reviewed by the local hospital. No patient identifiable information should be included. Any images of humans should come with consent.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. SQUIRE
7. Letters to the editor/short communication:
These are short pieces commenting on articles published through RMJ within the last 6 months (i.e. last two issues). These could include advocacy of a published RMJ article with a supporting argument or case etc. A rebuttal of a published RMJ article with supporting evidence would also be appropriate.
Letters to the editor only receive peer-review by 1 reviewer, and commentary is sought from the author of the original article. Final acceptance is at the discretion of the chief-editor.
Structure: Short, narrow, and focused
References: MDPI (Multidisciplinary Digital Publishing Institute) style, maximum five references (if commenting on a previous article from the RMJ then please reference this).
Word limit: 750 words (not including references)
Tables and figures: None
Writing style
The submitted manuscript should be referenced with MDPI referencing, and text style should be as per Oxford University Press Style Guide (Click here).
Title:
The title of the article should be concise and informative.
Abstract:
Maximum 250 words.
This is a synopsis, not an introduction to the article, which is informative and divided into five paragraphs with the following headings:
Background: a brief summation of previous research/opinions related to the body of work
Objective: stating the purposes/aims of the work; the research undergone, the hypothesis tested or the procedure evaluated.
Methods: briefly stating what was done and what materials were used, including the number of subjects, the methods to assess the data and to control bias.
Results: providing the findings of the study, including indicators of statistical significance, actual numbers, as well as percentages.
Conclusion: Summarizing in 1-2 sentences the conclusions of the work on the basis of the results. It emphasizes new and important aspects of the study or observations.
Keywords: Following the abstract. Up to five keywords or short phrases that assist indexers in cross-indexing the article. These should be terms found in the Medical Subject Heading (MeSH) index (https://www.ncbi.nlm.nih.gov/mesh/). These keywords are published with the article.
The main text:
The text of observational and experimental articles is divided into sections with the following headings:
Abbreviations should be spelled out the first time a term is given in the text.
Introduction: should always begin the text, and requires brevity and focus. Long articles may need subheadings to clarify their content. Different articles may be adapted according to their type. It conveys the nature and purpose of the work and quotes the relevant literature. Only strictly pertinent background information is necessary for understanding why the topic is important and why the study was undertaken. We suggest that the final paragraph clearly states the hypothesis or purpose of the study.
Methods: Details of clinical and technical procedures should follow the introduction. A clear description of the selection of the observational or experimental subjects should be given. The identification of all aspects of the study, it’s reasoning, and the related relevance should be explicitly justified. The criteria for subject exclusion or inclusion should be discussed. Identify in sufficient detail the methods, instrumentation, procedures, and all drugs and chemicals used (including generic names, doses, routes of administration) to allow other workers to reproduce the study.
Results: A logical sequence of presentation of results is required in the text; along with tables, and illustrations. Repetition of data from illustrations into the text should be avoided; however, emphasizing or summarizing important observations can be helpful.
Define all statistical terms including abbreviations and/or the most used symbols.
Any complications and/or unexpected results should be reported and the plausible explanation(s) given. The authors should also report losses to observation, such as dropouts from a clinical trial.
Statistics: Statistical methods should be described with enough details to enable a knowledgeable reader with access to the original data to verify the reported results.
Discussion: Use subheadings if required. Start the discussion with a clear response to the objectives of the study, but avoid the following:
Emphasize the new and important aspects of the study and the conclusions that follow from them. Avoid repetition of details included in other parts of the manuscript. This section requires that the limitations of the study are mentioned.
New hypotheses can be suggested when warranted, but they should be clearly labeled as such; and recommendations, when appropriate and needed, may be provided.
Acknowledgments: List all contributors who do not meet the criteria of authorship, such as those who provided purely technical help, writing assistance, or a department chair who provided only general support. Their respective contribution will be acknowledged as provided. Each contributor must have provided permission to be acknowledged.
Referencing - Citing articles and websites:
We strongly recommend the use of a referencing manager such as Mendeley. If using Mendeley, please submit manuscripts with the Mendeley links intact rather than plain text.
The journal uses the MDPI (Multidisciplinary Digital Publishing Institute) for referencing the citations. For guidance on using MDPI referencing click here
DOIs (Digital Object Identifiers) should be included in all references, where available. This can be done automatically by importing the articles into Mendeley and then referencing.
This journal recommends that authors use a reference manager (e.g., Zotero) to cite and manage references. MDPI is available on reference management software, such as Zotero. If citing unpublished work, the authors are responsible for obtaining permission and confirming the accuracy of the work.
Submitting the manuscript
Please have the following information and documentation ready when you submit your manuscript (in the submission form):
FORMATTING YOUR MANUSCRIPT - CHECKLIST
Please review a previously published article to gain a better understanding of how to format text. The journal does not have the capacity to format articles for authors. Articles which are not correctly formatted at submission will be returned to authors. Please go through the checklist below:
Three documents minimum should be submitted:
1. Accompanying letter: summarising the work and the importance of the manuscript. This should also state that the work is original (not submitted elsewhere) and that all named authors have taken responsibility for the work.
2. Author details: A separate Microsoft Word file with all the authors’ names preceded by initials of their first names, affiliated institutions, as well as the name and full address of the corresponding author should be provided. Please ensure that all names and affiliations are correctly spelled as this is how they will appear in final print. Though not a requirement, we strongly recommend that all authors register with http://orcid.org and provide their ORCID addresses within this document. These will then be included in the article.
3. Manuscript: A Microsoft Word file without any author information within the document. Single spaced. Size 10 Calibri Light font . Tables should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
4. Figures: When figures are used, they should be submitted as a separate high-quality image file (e.g., TIFF, JPEG, etc.). Please note that if you have taken a figure from another source you will need to ensure that you have permission to use it from the Copyright holder. Alternatively you will need to create your own distinct figure. Our journal is published entirely online therefore color figures can be provided.
Authorship
We adhere to the criteria of the ICMJE (International Committee of Medical Journal Editors). Please consult the ICMJE website for more information. We require confirmation that ALL authors have read, understood, and are bound to the journal rules.
Authorship is constituted by:
· Substantial contributions to the conception OR design of the work; OR the acquisition, analysis, OR interpretation of data for the work; AND
· Drafting the work or revising it critically for important intellectual content; AND
· Final approval of the version to be published; AND
· Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
NB: The RMJ does not publish papers that make use of data, infrastructure, or personnel in a foreign country without involving at least one scientist from that foreign country as an author.
Any involvement not included in these criteria would be considered as ‘’Contributorship‘’.
Before assessment of the authorship declaration, ALL authors must be aware of it and agree to be listed.
All authors should agree that no person who meets the criteria (above) has been omitted and that conflicts of interest are acknowledged and fully declared.
For more details on inappropriate types of authorship have been described, including guest authorship, honorary or gift authorship, and ghost authorship see the guidance from the Council of Science Editors (click here)
Conflict of interest
The ICMJE (International Committee of Medical Journal Editors) give guidance on conflicts of interest (click here)
As per ICMJE guidance articles should be published with a statement of any conflict of interest form, declaring:
Type-editing and copy-editing
Articles may be sent to “type editors” at the discretion of the editorial team. Type editors are proficient/native English speakers who will amend the manuscript to enhance the grammar and spelling. Type-edited amendments are sent to the authors in Tracked changes format. Type editing does not incur a charge on the authors.
Peer-reviewers are also asked to add direct copy-editing feedback in the manuscript to improve the quality of the manuscript. The amended manuscript will be given to authors with anonymous tracked changes
Peer Review
All original articles, case reports, and review articles are sent for peer review. Commissioned articles are also subject to standard peer-review procedures. Peer review aims to ensure that all authors receive feedback as well as the decision regarding their submission. A minimum of two experts reviews submitted manuscripts. The review follows a double-blind peer review process meaning that reviewers are not given any identifying features of the authors of the manuscript. Authors are provided with feedback from the peer review, and this is anonymized.
Peer reviewers have five possible options:
1.Accept in current form
2.Accept after minor revision (by the journal editorial team)
3.Reject initial submission for minor revision: asking the author(s) to make minor revisions and resubmit
4.Reject initial submission for major revision: asking the author(s) to make significant revisions and resubmit
5.Reject initial submission entirely: with no opportunity for resubmission.
Acceptance/Rejection
Decisions of whether to accept or reject a manuscript are based on the reviews given by peer-reviewers. The peer-review form is available below as an appendix. Many authors find it helpful to read this and rework their manuscript using the information held in this peer-review form.
Editorial expert board (EEB)
The journal uses a double-blind peer review process for the review of manuscripts submitted for publication in the journal.
Articles Approval and Publication: Every article shall be reviewed by a minimum of two independent peer-reviewers before they are accepted (except letters to the editor). The journal uses a double-blind peer-review process. In case the committee believes that the article would require a substantial redrafting, suggestions shall be made and the reviewers shall proceed with a review of both style and language. Articles shall remain the authors' property even after publication.
Online Open access
For any published article, the author shall access the entire content as published through the journal online web publisher: www.bioline.org.br/rw and https://www.ajol.info/index.php/rmj
The journal considers the following types of articles:
1. Original research:
These should be pieces of original research that have not been published in other peer-reviewed journals
Structure: The manuscript should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods, Results, Discussion, Conclusion (see writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 3500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum four produced to high quality.
Tables and figures should be presented in the text in Results section of the manuscript and should be single-spaced.
Trial registration and Institutional Review Board (IRB): The IRB reference code should be reported in all manuscripts as the last line of the manuscript abstract. The trial registration number should be included where relevant.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. STROBE
2. Reviews:
We accept the following types of review articles:
i. Systematic reviews and realist reviews
ii. In-depth critical reviews in areas where these are more appropriate; e.g., coverage of issues recently updated. For non-commissioned reviews, the editorial board recommend contacting us to discuss the concept before investing time into the manuscript
iii. Best-evidence topics (BETs). These are not systematic reviews but critical review articles of evidence related to a specific clinical question. Contact Dr. Peter Cartledge ([email protected]) for more details and a Word template.
Structure: the manuscript should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods (including search strategy where relevant), Results, Discussion, Conclusion (see writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 4500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum four produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
3. Case report or case studies:
These should be a well-described case for educational purposes and/or to extend scientific knowledge regarding unique clinical cases. These can contain unusual features of disease, treatment, transmission and/or control.
Structure: the manuscript should be divided into the following sections: Keywords, Short running Title, Abstract. Introduction, Case(s), Conclusions (see writing rules & styles below for more information).
Abstract: A short abstract (maximum 100 words) can be provided using the following two subheadings: Case, Discussion.
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 1500-2000 words (not including, title, tables, abstract and/or references)
Tables: Maximum 2
Figures: Maximum three produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
Consent: Informed consent must be documented. A signed written consent from the patient should be provided for any potentially identifiable images.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. CARE checklist
Clinical images:
Images are accepted and encouraged with the same instructions as case-reports and therefore they should include a description of the case (as above). Written consent should be obtained from patients and a copy of the consent form included with the submission.
4. Methodology articles:
New experimental methods, tests, techniques, or procedures. These should be completely new (novel) or improved, and/or compared with different methods
Structure: should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methodology, Conclusions (see Writing rules & styles below for more information)
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on number of references
Word limit: 3500 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum 4 produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single spaced, each table and figure on a separate page, starting with a “page break”.
5. Editorial/Opinion articles:Editorials are short opinion papers.
These articles might mostly be object to critical and opened discussion categorized as ‘’commentaries‘’. These are normally commissioned and should always be discussed with the editorial board prior to starting work on the manuscript.
Structure: Short, narrow and focused
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on number of references
Word limit: 2500 words (including, references)
Tables and figures: Maximum 2 (e.g. 1 Table & 1 figure) produced to high quality. Tables and figures should be inserted in the text where they would appear in the manuscript. Do not attach as appendices.
6. Quality improvement project
Quality Improvement projects that have not been published in other peer-reviewed journals:
These should be divided into the following sections: Abstract (max 250 words), Keywords, Short running Title, Introduction, Methods, Results, Discussion, Conclusion (see writing rules & styles below for more information). If a traditional IMRaD structure is not used then authors may choose to present in a PDSA (Plan, Do, Study, Act) format
Methodology: How was the QI project undertaken
Results: What was found and how did care improve
Discussion: What are the implications and potential for wider application.
References: MDPI (Multidisciplinary Digital Publishing Institute) style, no limit on the number of references
Word limit: 3000 words (not including, title, abstract and/or references)
Tables: Maximum 4
Figures: Maximum 4 - produced to high quality.
Tables and figures should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
Trial registration and Institutional Review Board (IRB): Quality Improvement projects do not require IRB review for publication. However authors should state that the project has been reviewed by the local hospital. No patient identifiable information should be included. Any images of humans should come with consent.
Checklist: Prior to submitting, authors should review and amend their manuscript to ensure that the content complies with the relevant checklist. Authors should state the name of the checklist they have used and reference it in the body of the manuscript. E.g. SQUIRE
7. Letters to the editor/short communication:
These are short pieces commenting on articles published through RMJ within the last 6 months (i.e. last two issues). These could include advocacy of a published RMJ article with a supporting argument or case etc. A rebuttal of a published RMJ article with supporting evidence would also be appropriate.
Letters to the editor only receive peer-review by 1 reviewer, and commentary is sought from the author of the original article. Final acceptance is at the discretion of the chief-editor.
Structure: Short, narrow, and focused
References: MDPI (Multidisciplinary Digital Publishing Institute) style, maximum five references (if commenting on a previous article from the RMJ then please reference this).
Word limit: 750 words (not including references)
Tables and figures: None
Writing style
The submitted manuscript should be referenced with MDPI referencing, and text style should be as per Oxford University Press Style Guide (Click here).
Title:
The title of the article should be concise and informative.
Abstract:
Maximum 250 words.
This is a synopsis, not an introduction to the article, which is informative and divided into five paragraphs with the following headings:
Background: a brief summation of previous research/opinions related to the body of work
Objective: stating the purposes/aims of the work; the research undergone, the hypothesis tested or the procedure evaluated.
Methods: briefly stating what was done and what materials were used, including the number of subjects, the methods to assess the data and to control bias.
Results: providing the findings of the study, including indicators of statistical significance, actual numbers, as well as percentages.
Conclusion: Summarizing in 1-2 sentences the conclusions of the work on the basis of the results. It emphasizes new and important aspects of the study or observations.
Keywords: Following the abstract. Up to five keywords or short phrases that assist indexers in cross-indexing the article. These should be terms found in the Medical Subject Heading (MeSH) index (https://www.ncbi.nlm.nih.gov/mesh/). These keywords are published with the article.
The main text:
The text of observational and experimental articles is divided into sections with the following headings:
- Introduction
- Methods
- Results
- Discussion
- Conclusion
Abbreviations should be spelled out the first time a term is given in the text.
Introduction: should always begin the text, and requires brevity and focus. Long articles may need subheadings to clarify their content. Different articles may be adapted according to their type. It conveys the nature and purpose of the work and quotes the relevant literature. Only strictly pertinent background information is necessary for understanding why the topic is important and why the study was undertaken. We suggest that the final paragraph clearly states the hypothesis or purpose of the study.
Methods: Details of clinical and technical procedures should follow the introduction. A clear description of the selection of the observational or experimental subjects should be given. The identification of all aspects of the study, it’s reasoning, and the related relevance should be explicitly justified. The criteria for subject exclusion or inclusion should be discussed. Identify in sufficient detail the methods, instrumentation, procedures, and all drugs and chemicals used (including generic names, doses, routes of administration) to allow other workers to reproduce the study.
Results: A logical sequence of presentation of results is required in the text; along with tables, and illustrations. Repetition of data from illustrations into the text should be avoided; however, emphasizing or summarizing important observations can be helpful.
Define all statistical terms including abbreviations and/or the most used symbols.
Any complications and/or unexpected results should be reported and the plausible explanation(s) given. The authors should also report losses to observation, such as dropouts from a clinical trial.
Statistics: Statistical methods should be described with enough details to enable a knowledgeable reader with access to the original data to verify the reported results.
Discussion: Use subheadings if required. Start the discussion with a clear response to the objectives of the study, but avoid the following:
- Unqualified statement not completely supported by the data
- Statement on economic benefits and costs unless the report includes economic data and analyses
- Claim of priority and alluding to work that has not been completed.
Emphasize the new and important aspects of the study and the conclusions that follow from them. Avoid repetition of details included in other parts of the manuscript. This section requires that the limitations of the study are mentioned.
New hypotheses can be suggested when warranted, but they should be clearly labeled as such; and recommendations, when appropriate and needed, may be provided.
Acknowledgments: List all contributors who do not meet the criteria of authorship, such as those who provided purely technical help, writing assistance, or a department chair who provided only general support. Their respective contribution will be acknowledged as provided. Each contributor must have provided permission to be acknowledged.
Referencing - Citing articles and websites:
We strongly recommend the use of a referencing manager such as Mendeley. If using Mendeley, please submit manuscripts with the Mendeley links intact rather than plain text.
The journal uses the MDPI (Multidisciplinary Digital Publishing Institute) for referencing the citations. For guidance on using MDPI referencing click here
DOIs (Digital Object Identifiers) should be included in all references, where available. This can be done automatically by importing the articles into Mendeley and then referencing.
This journal recommends that authors use a reference manager (e.g., Zotero) to cite and manage references. MDPI is available on reference management software, such as Zotero. If citing unpublished work, the authors are responsible for obtaining permission and confirming the accuracy of the work.
Submitting the manuscript
Please have the following information and documentation ready when you submit your manuscript (in the submission form):
FORMATTING YOUR MANUSCRIPT - CHECKLIST
Please review a previously published article to gain a better understanding of how to format text. The journal does not have the capacity to format articles for authors. Articles which are not correctly formatted at submission will be returned to authors. Please go through the checklist below:
- Font: Calibri light – size 10 font, single spacing, throughout the body of the text
- Title in bold, small case (not capitals)
- Subheadings (e.g. introduction) should be bold capitals
- Further subheadings should be bold, small case
- Ensure style is: No Spacing
- Single column (i.e. don’t put text into two columns)
- Text should be justified
- Tables
- Calibri light, Font size 9
- No vertical lines. Horizontal lines in table can be removed (see recently published articles)
- No table should be larger then a single A4 page as it won’t be possible to fit on one page of the publication
- Check keywords are MeSH (click here) and put semi-colons (;) between each keyword
- Referencing should be MDPI – Ideally authors should submit with Mendeley references active as this will save the author additional work later
- Each author's name, address, and e-mail address, if possible should be included in a separate author details letter
- The name of the author who will deal with correspondence and proofs; His/Her email address must be submitted with the manuscript.
- For animal or human studies that involve data collected actively and purposely, we require a signed statement from the corresponding or primary author that ethical approval was granted by an appropriate institution. Institutional Review Board (IRB), or equivalent, letters of approval should be submitted with the manuscript. IRB, or equivalent, reference codes should be included in the manuscript.
Three documents minimum should be submitted:
1. Accompanying letter: summarising the work and the importance of the manuscript. This should also state that the work is original (not submitted elsewhere) and that all named authors have taken responsibility for the work.
2. Author details: A separate Microsoft Word file with all the authors’ names preceded by initials of their first names, affiliated institutions, as well as the name and full address of the corresponding author should be provided. Please ensure that all names and affiliations are correctly spelled as this is how they will appear in final print. Though not a requirement, we strongly recommend that all authors register with http://orcid.org and provide their ORCID addresses within this document. These will then be included in the article.
3. Manuscript: A Microsoft Word file without any author information within the document. Single spaced. Size 10 Calibri Light font . Tables should be presented at the end of the manuscript after the references, single-spaced, each table and figure on a separate page, starting with a "page break."
4. Figures: When figures are used, they should be submitted as a separate high-quality image file (e.g., TIFF, JPEG, etc.). Please note that if you have taken a figure from another source you will need to ensure that you have permission to use it from the Copyright holder. Alternatively you will need to create your own distinct figure. Our journal is published entirely online therefore color figures can be provided.
Authorship
We adhere to the criteria of the ICMJE (International Committee of Medical Journal Editors). Please consult the ICMJE website for more information. We require confirmation that ALL authors have read, understood, and are bound to the journal rules.
Authorship is constituted by:
· Substantial contributions to the conception OR design of the work; OR the acquisition, analysis, OR interpretation of data for the work; AND
· Drafting the work or revising it critically for important intellectual content; AND
· Final approval of the version to be published; AND
· Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
NB: The RMJ does not publish papers that make use of data, infrastructure, or personnel in a foreign country without involving at least one scientist from that foreign country as an author.
Any involvement not included in these criteria would be considered as ‘’Contributorship‘’.
Before assessment of the authorship declaration, ALL authors must be aware of it and agree to be listed.
All authors should agree that no person who meets the criteria (above) has been omitted and that conflicts of interest are acknowledged and fully declared.
For more details on inappropriate types of authorship have been described, including guest authorship, honorary or gift authorship, and ghost authorship see the guidance from the Council of Science Editors (click here)
Conflict of interest
The ICMJE (International Committee of Medical Journal Editors) give guidance on conflicts of interest (click here)
As per ICMJE guidance articles should be published with a statement of any conflict of interest form, declaring:
- Authors’ conflicts of interest; and
- Sources of support for the work, including sponsor names along with explanations of the role of those sources if any in study design; collection, analysis, and interpretation of data; writing of the report; the decision to submit the report for publication; or a statement declaring that the supporting source had no such involvement; and
- Whether the authors had access to the study data, with an explanation of the nature and extent of access, including whether access is on-going.
Type-editing and copy-editing
Articles may be sent to “type editors” at the discretion of the editorial team. Type editors are proficient/native English speakers who will amend the manuscript to enhance the grammar and spelling. Type-edited amendments are sent to the authors in Tracked changes format. Type editing does not incur a charge on the authors.
Peer-reviewers are also asked to add direct copy-editing feedback in the manuscript to improve the quality of the manuscript. The amended manuscript will be given to authors with anonymous tracked changes
Peer Review
All original articles, case reports, and review articles are sent for peer review. Commissioned articles are also subject to standard peer-review procedures. Peer review aims to ensure that all authors receive feedback as well as the decision regarding their submission. A minimum of two experts reviews submitted manuscripts. The review follows a double-blind peer review process meaning that reviewers are not given any identifying features of the authors of the manuscript. Authors are provided with feedback from the peer review, and this is anonymized.
Peer reviewers have five possible options:
1.Accept in current form
2.Accept after minor revision (by the journal editorial team)
3.Reject initial submission for minor revision: asking the author(s) to make minor revisions and resubmit
4.Reject initial submission for major revision: asking the author(s) to make significant revisions and resubmit
5.Reject initial submission entirely: with no opportunity for resubmission.
Acceptance/Rejection
Decisions of whether to accept or reject a manuscript are based on the reviews given by peer-reviewers. The peer-review form is available below as an appendix. Many authors find it helpful to read this and rework their manuscript using the information held in this peer-review form.
Editorial expert board (EEB)
The journal uses a double-blind peer review process for the review of manuscripts submitted for publication in the journal.
Articles Approval and Publication: Every article shall be reviewed by a minimum of two independent peer-reviewers before they are accepted (except letters to the editor). The journal uses a double-blind peer-review process. In case the committee believes that the article would require a substantial redrafting, suggestions shall be made and the reviewers shall proceed with a review of both style and language. Articles shall remain the authors' property even after publication.
Online Open access
For any published article, the author shall access the entire content as published through the journal online web publisher: www.bioline.org.br/rw and https://www.ajol.info/index.php/rmj
Policy Timeline:
Reviewed by editorial board: February 2020
Approved: 1st March 2022 (Senior editorial board meeting)
Review date: 1st March 2024
Reviewed by editorial board: February 2020
Approved: 1st March 2022 (Senior editorial board meeting)
Review date: 1st March 2024